Primary Objective
The purpose of this study is to evaluate the efficacy and safety of zanidatamab plus CisGem (Cisplatin and Gemcitabine) with or without the addition of a programmed death protein 1/ligand-1 (PD-1/L1) inhibitor (physician’s choice of either Durvalumab or Pembrolizumab, where approved under local regulations) as first line of treatment for participants with human epidermal growth factor receptor 2 (HER2)-positive biliary tract cancer.
Study Design
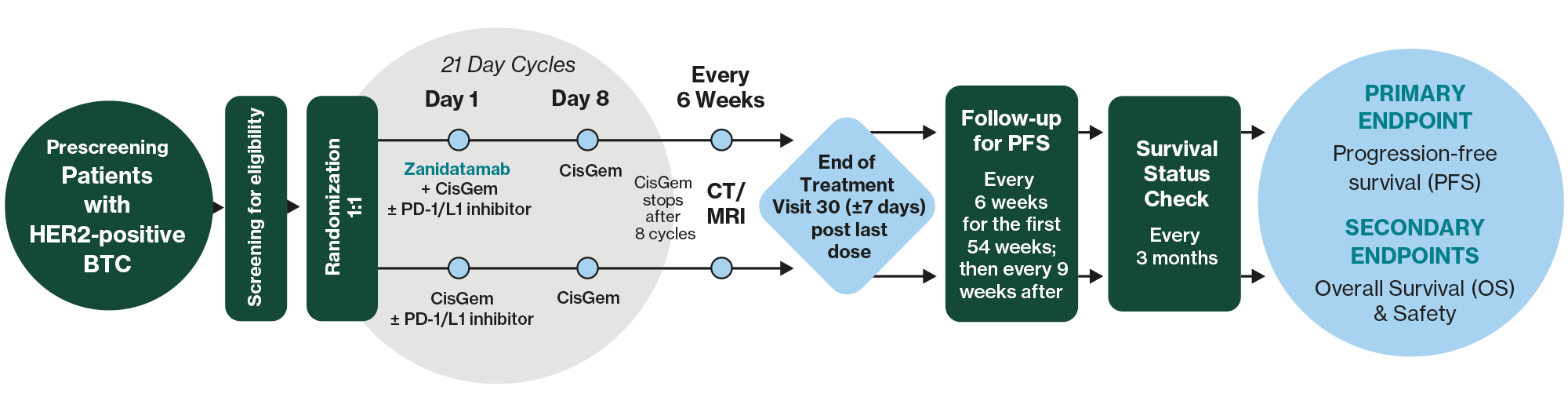
Zanidatamab
A novel bispecific antibody that simultaneously binds to two distinct domains on HER2, promoting receptor cross-linking and is believed to drive multiple mechanisms of action including HER2 internalization, signal inhibition, and the induction of immune-mediated effects.
Status
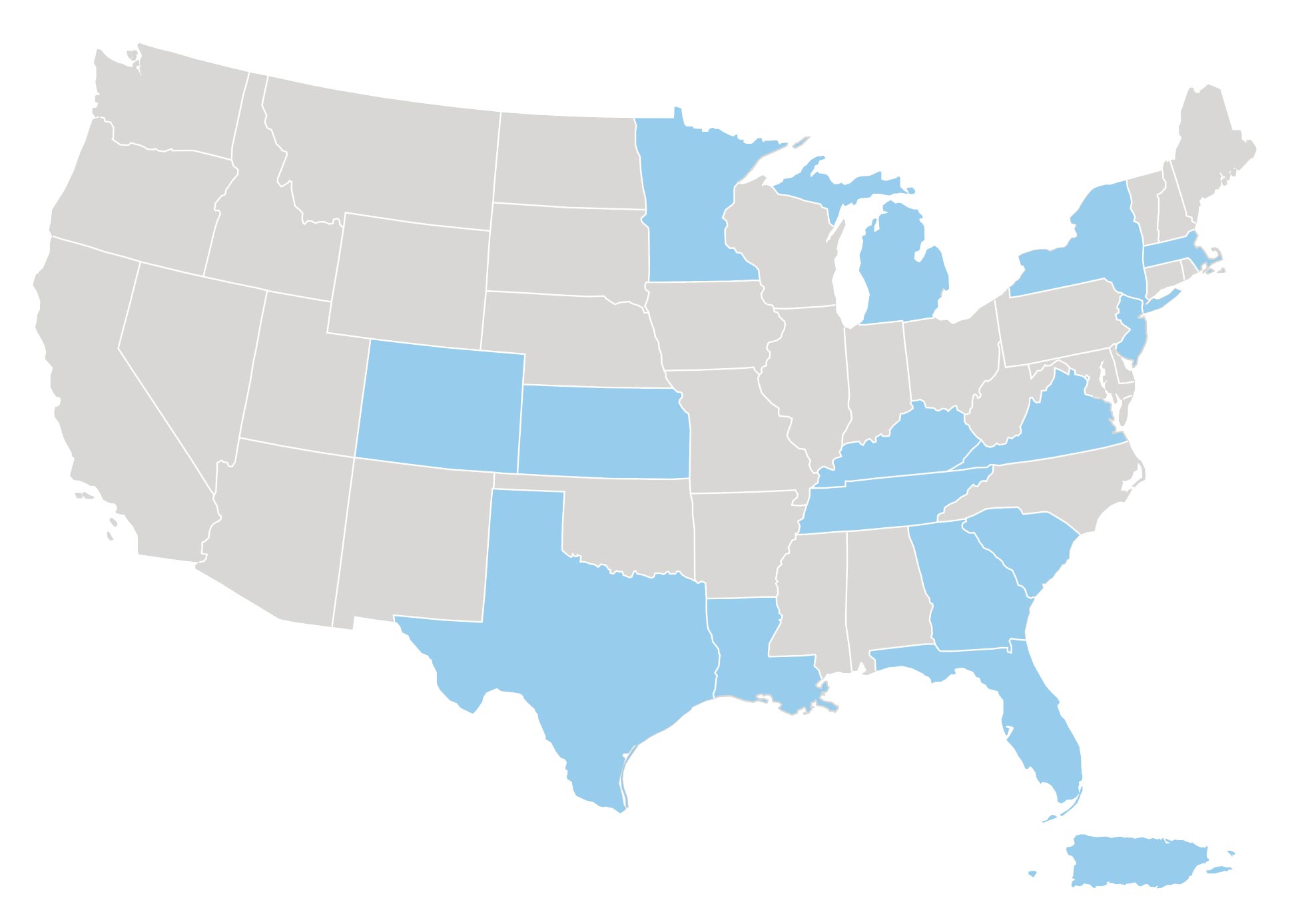
Recruiting globally in the following countries: Argentina, Belgium, Brazil, Canada, Chile, China, Czech Republic, Finland, France, Germany, India, Israel, Italy, Japan, Portugal, Romania, Serbia, S. Korea, Spain, Sweden, Taiwan, Turkey, United Kingdom, and USA.


Study Population
Patients 18 years of age with locally advanced unresectable or metastatic HER2-positive BTC and not eligible for curative resection, transplantation, or ablative therapies, and who have not had any therapy or who have just started therapy.
Key Inclusion Criteria
- Participants must have a confirmed diagnosis of HER2-positive Biliary Tract Cancer, including Gallbladder Cancer, Intrahepatic Cholangiocarcinoma, or Extrahepatic Cholangiocarcinoma that cannot be effectively treated with surgery or other localized (in the body) treatments such as radiation therapy or direct tumor destruction.
- Participants may only have received up to 2 prior treatment cycles of standard therapy.
- Participants must be male or female over 18 years of age (or the legal age of adulthood per country-specific regulations).
- Females of childbearing potential and males with a partner of childbearing potential must be willing to use 2 methods of birth control.
See full list of inclusion criteria
- Histologically- or cytologically-confirmed BTC, including GBC, ICC, or ECC.
- Locally advanced unresectable or metastatic BTC and not eligible for curative resection, transplantation, or ablative therapies.
- Received no more than 2 cycles of systemic therapy which is limited to CisGem with or without a PD-1/L1 inhibitor (physician’s choice of durvalumab or pembrolizumab, where approved under local regulations) for advanced unresectable or metastatic disease. Participants who have received prior adjuvant or neoadjuvant treatment (including investigational products) for earlier stage disease are permitted as long as therapy was completed more than 6 months prior to expected date of C1D1.
- HER2-positive disease (defined as IHC 3+; or IHC 2+/ ISH+) by IHC and ISH assay (in participants with IHC 2+ tumors) at a central laboratory on new biopsy tissue or archival tissue from the most recent biopsy (see Section 7.1). Note that fine needle aspirates (FNAs; which obtain only cytology samples), cytology samples, brushings, and biopsies from sites of bone metastases are not acceptable. Biopsies obtained with a needle that provides intact tissue samples may be acceptable. Testing may occur with tissue obtained at any time after diagnosis of BTC and before expected date of randomization.
- When the central laboratory is available, samples must be sent to the central laboratory prior to randomization for determination of HER2 status. If it has been confirmed with the medical monitor that a central laboratory is not available, sites may use local testing for HER2 to enroll participants with IHC 3+ tumors. In this case, samples still must be sent to the central laboratory for confirmatory analyses once the central laboratory becomes available.
- Assessable (measurable or non-measurable) disease as defined by RECIST 1.1, per investigator assessment.
- Male or female ≥ 18 years or age (or the legal age of adulthood per country-specific regulations).
- Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
- Adequate hematologic function as follows:
- Absolute neutrophil count (ANC) ≥ 1.5 × 109/L
- Platelet count ≥ 100 × 109/L, not requiring transfusion support
- Hemoglobin (Hgb) ≥ 9 g/dL (participants with chronic anemia that is supported by intermittent red blood cell transfusions are eligible)
- Adequate hepatic function, as defined by both:
- Aspartate aminotransferase (AST) ≤ 3 × upper limit of normal (ULN), and alanine aminotransferase (ALT) ≤ 3 × ULN. For participants with liver involvement, AST and ALT ≤ 5 × ULN is acceptable.
- Total bilirubin ≤ 1.5 × ULN, or ≤ 3 × ULN for participants with Gilbert’s disease
- Adequate renal function, as defined by estimated glomerular filtration rate (GFR) > 50 mL/min per local institutional standard method.
- Left ventricular ejection fraction (LVEF) ≥ 50% as determined by either echocardiogram or multiple gated acquisition scan (MUGA).
- Females of childbearing potential must have a negative serum/plasma or urine beta human chorionic gonadotropin (β-hCG) pregnancy test result within 3 days (72 hours) prior to expected date of C1D1. Females with false positive results can be enrolled if subsequent serum/plasma testing is negative.
- Females of childbearing potential and males with a partner of childbearing potential must be willing to use 2 methods of birth control with a failure rate of less than 1% per year (HMA-CTCG, 2024) during the study and for 14 months after the last dose of cisplatin, 6 months after the last dose of gemcitabine, 4 months after the last dose of zanidatamab, 4 months after the last dose of pembrolizumab, and 3 months after the last dose of durvalumab.
- Females must agree to not donate oocytes starting at screening and throughout the study period, and for at least 14 months after the last dose of cisplatin, 6 months after the last dose of gemcitabine, 4 months after the last dose of zanidatamab, 4 months after the last dose of pembrolizumab, and 3 months after the last dose of durvalumab.
- Males must agree to use condoms and not to donate sperm starting at screening and throughout the study period, and for at least 11 months after the last dose of cisplatin, 4 months after the last dose of zanidatamab, and 6 months after the last dose of gemcitabine.
- Participant has life expectancy of greater than 3 months, in the opinion of the investigator.
- The participant must provide written informed consent. Participants who elect to be pre-screened for HER2 status must provide a separate written informed consent for collection, storage, and analysis of the tumor tissue
Key Exclusion Criteria
- Participants may not have received prior treatment with a HER2-targeted agent or checkpoint inhibitors. The only exception is if they have received two or fewer doses of durvalumab or pembrolizumab as part of the treatment that is allowed prior to study entry (described in the inclusion criteria).
- Participants must not use high dose systemic corticosteroids or other medications that suppress the immune system. There are some exceptions to this, please consult with your doctor about what is allowed.
- Participants must not have brain metastases.
- Participants may not have severe chronic or active infections, a history of allogeneic organ transplantation, active autoimmune disease that has required systemic treatment in past 2 years, or interstitial lung disease or non-infectious pneumonitis.
- Participants may not have participated in another clinical trial with an investigational medicinal product within the last 3 months.
- Females who are breastfeeding are not eligible to participate.
- Participants may not have any other medical, social, or psychosocial factors that, in the opinion of the investigator, could impact safety or compliance with study procedures.
See fill list of exclusion criteria
- Prior treatment with a HER2-targeted agent, with the exception of participants who completed HER2-targeted treatment for breast cancer > 5 years prior to their diagnosis of BTC.
- Prior treatment with checkpoint inhibitors, other than durvalumab or pembrolizumab as part of the up to 2 cycles of systemic therapy allowed prior to expected date of C1D1 per Inclusion Criterion 3. Exclusionary checkpoint inhibitors include but are not limited to other anti-PD-1, anti-PD-L1, anti-cytotoxic T lymphocyte-associated antigen (CTLA)-4 antibodies.
- The following BTC histologic subtypes are excluded: small cell cancer, neuroendocrine tumors, lymphoma, sarcoma, mixed tumor histology, and mucinous cystic neoplasms detected in the biliary tract region.
- Received radiotherapy within 2 weeks of expected date of C1D1.
- Had major surgery within 4 weeks of expected date of C1D1.
- Total lifetime load of anthracycline exceeding 360 mg/m2 doxorubicin or equivalent.
- Use of systemic corticosteroids administered at doses equivalent to > 10 mg per day of prednisone within 2 weeks of expected date of C1D1. Topical, ocular, intra-articular, intranasal, and/or inhalation corticosteroids are permitted.
- Brain metastases: Untreated central nervous system (CNS) metastases, symptomatic CNS metastases, or radiation treatment for CNS metastases within 4 weeks of expected date of C1D1. Stable, treated brain metastases are allowed (defined as participants who are off steroids and anticonvulsants and are neurologically stable with no evidence of radiographic progression for at least 4 weeks at the time of screening).
- Known history of or ongoing leptomeningeal disease (LMD). Participants will be eligible if LMD has been reported radiographically but is not suspected clinically by the investigator, and the participant does not have neurological symptoms of LMD.
- Poorly controlled seizures in the judgment of the investigator.
- Grade 2 or greater peripheral neuropathy that is related to prior cancer therapy.
- Concurrent uncontrolled or active hepatobiliary disorders or untreated or ongoing complications after laparoscopic procedures or stent placement, including but not limited to active cholangitis, unresolved biliary obstruction, infected biloma, or abscess. Any complications must be resolved at least 2 weeks prior to expected date of C1D1.
- Prior or concurrent invasive malignancy whose natural history or treatment has, in the opinion of the investigator or medical monitor, the potential to interfere with the safety or efficacy assessment of the investigational regimen.
- Severe chronic or active infections requiring systemic parenteral antibacterial, antifungal or antiviral therapy; or any other potentially life-threatening viral or bacterial infection (participants on oral antibiotics must complete the planned course of treatment prior to expected date of C1D1).
- Active hepatitis, including the following:
- Acute or chronic hepatitis B (Exception: Participants who are hepatitis B surface antigen [HbsAg] positive are eligible if they have hepatitis B virus (HBV) DNA less than 500 IU/mL or 2,500 copies/mL). Note: Participants with detectable HbsAg or detectable HBV DNA should be managed per institutional or local standards. Participants beginning antiviral agents at screening should be treated for > 2 weeks prior to expected date of C1D1.
- Infection with hepatitis C (Exceptions: [i] Participants who have no history of curative viral treatment and are documented to be viral load negative are eligible; [ii] Participants who have completed curative viral therapy ≥ 12 weeks or on concurrent hepatitis C virus treatment prior to expected date of C1D1, and viral load is negative are eligible.)
- Infection with human immunodeficiency virus (HIV)-1 or HIV-2. (Exception: Participants with well-controlled HIV [ie, CD4 > 350/mm3 and undetectable viral load] are eligible.)
- Active tuberculosis.
- History of allogeneic organ transplantation.
- Active autoimmune disease that has required systemic treatment in past 2 years (i.e., with use of disease-modifying agents, corticosteroids, or immunosuppressive drugs) with the exception of:
- Vitiligo or alopecia
- Endocrine disorders stable on replacement therapy (thyroxine, insulin, etc)
- Chronic skin conditions that do not require systemic therapy
- Celiac disease controlled by diet alone
- Primary sclerosing cholangitis
Note: Replacement therapy (eg, thyroxine, insulin, or physiologic corticosteroid replacement therapy for adrenal or pituitary insufficiency, etc) is not considered a form of systemic treatment of the autoimmune disease and is allowed.
- History of life-threatening hypersensitivity to monoclonal antibodies or to recombinant proteins or excipients in the drug formulation of any of the agents in the trial (cisplatin, gemcitabine, the selected PD-1/L1 inhibitor, or zanidatamab).
- Known hypersensitivity to any components of the combination therapy.
- Ongoing, clinically significant toxicity (Grade 2 or higher) associated with prior cancer therapies, with the exception of alopecia.
- Clinically significant cardiac disease, such as ventricular arrhythmia requiring therapy, uncontrolled hypertension or any history of symptomatic congestive heart failure (CHF). Participants with known myocardial infarction or unstable angina within 6 months (180 days) prior to expected date of C1D1 are also excluded. Previous anticancer therapy-related CHF must have been ≤ Grade 1 at the time of occurrence and must have completely resolved.
- History of interstitial lung disease or non-infectious pneumonitis.
- History of active primary immunodeficiency.
- Participation in another clinical trial with an investigational medicinal product within the last 3 months (90 days).
- Acute or chronic uncontrolled pancreatitis or Child-Pugh Class C liver disease.
- Females who are breastfeeding or pregnant, and females and males planning a pregnancy.
- Any other medical, social, or psychosocial factors (eg, hearing impairment) that, in the opinion of the investigator, could impact safety or compliance with study procedures.
- Use of prophylactic phenytoin.
Frequently Asked Questions
What is a study?
A study (also called a clinical trial) is a medical investigation that helps to answer important questions about an investigational medication, including if the medication works for a certain condition and how safe and tolerable the medication is. All medications must be tested in clinical research studies before they can be approved by regulatory authorities and prescribed to patients.
What is a study?
Why are studies important?
What is the purpose of the study?
The purpose of this study is to find out if the study medicine works, and if it is safe and effective to use in adults with HER2-positive Biliary Tract Cancer.
Will compensation for time and travel be provided?
Is there a cost to participate?
- You will receive study-related care from a team of experienced doctors and nurses throughout the study.
- All study-related visits, tests, assessments, and investigational medication will be provided at no cost to you.
What else do I need to consider?
Where are the study sites located?
The Study Is Taking Place Now!
Investigator Locator
Contact a participating site to learn if this trial may be appropriate for you or your loved ones. Please reach out to us at ClinicalTrialDisclosure@jazzpharma.com to learn more about our sites near you.
The University of Kansas Cancer Center - Westwood
Atlantic Health System/Morristown Medical Center
Virginia Commonwealth University, VCU Health
Ochsner Clinic Foundation
Winship Cancer Institute
Norton Cancer Institute
SITE CONTACT:
Michael Driscoll, MD
Principal Investigator
Email:
GI-NCIResearch@nortonhealthcare.org
Phone:
+1 502-629-2500
University of Michigan
SITE CONTACT:
Vaibhav Sahai
Principal Investigator
UM Cancer Answerline Nurses
Study Coordinator
Email:
CancerAnswerLine@med.umich.edu
Phone:+1 800-865-1125
Henry Ford Health System
Contact information: clinicaltrialdisclosure@jazzpharma.com
James J. Harding Memorial Sloan Kettering Cancer
SITE CONTACT:
James J. Harding, MD
Principal Investigator
Raymond Teer
Study Coordinator
Email: teerr@mskcc.org
Amin Yaqubie
Research Manager
Email: yaqubiea@mskcc.org
Elizabeth Warner
Research Manager
Email: warnere@mskcc.org
Phone:
+1 332-456-7016
Laura and Isaac Perlmutter Cancer Center at NYU Langone - Ambulatory Care Center
SITE CONTACT:
Spencer
Principal Investigator
clinicaltrialdisclosure@jazzpharma.com
Tufts Medical Center
SITE CONTACT:
Nugent
Principal Investigator
clinicaltrialdisclosure@jazzpharma.com
Saint Francis Cancer Center
SITE CONTACT:
Yang
Principal Investigator
clinicaltrialdisclosure@jazzpharma.com
Sarah Cannon Research Institute (SCRI) Oncology
SITE CONTACT:
Meredith Pelster, MD
Principal Investigator
Phone:
+1 844-482-4812
James J. Harding Memorial Sloan Kettering Cancer
Puerto Rico Medical Center
Second Floor, Barrio Monacillos
Rio Piedras, Puerto Rico, USA 00935
SITE CONTACTS:
Karina Arocho-Gonzalez, MD
Principal Investigator
Email:karina.arocho@panoncologytrials.com
Isamar Alicea
Study Coordinator
Email:isamar.alicea@panoncologytrials.com
Phone: +1 787-407-3333
Website: panoncologytrials.com
AdventHealth Hematology and Oncology
Location: Orlando, Florida, US 32804
Contact information: clinicaltrialdisclosure@jazzpharma.com
Rocky Mountain Cancer Centers, LLP
Location: Lone Tree, Colorado, US 80124
Contact information: clinicaltrialdisclosure@jazzpharma.com
Texas Oncology - DFW
Contact information: clinicaltrialdisclosure@jazzpharma.com
University of Texas Southwestern Medical Center
The University of Texas MD Anderson Cancer Center
Contact information: clinicaltrialdisclosure@jazzpharma.com
Minnesota Oncology Hematology, P.A.
Contact information: clinicaltrialdisclosure@jazzpharma.com
